SOLVED: 'Anideal gas mixture contains 50.0% helium; 30.0% methane, and 20.0% nitrogen by volume at 1.50atm absolute and 80.08C (a) Calculate the partial pressure of each component: Helium: atm Methane: atm Nitrogen:

Question Video: Determining the Mole Fraction of a Gas Given the Mole Fraction of the Other Gas in the Mixture | Nagwa

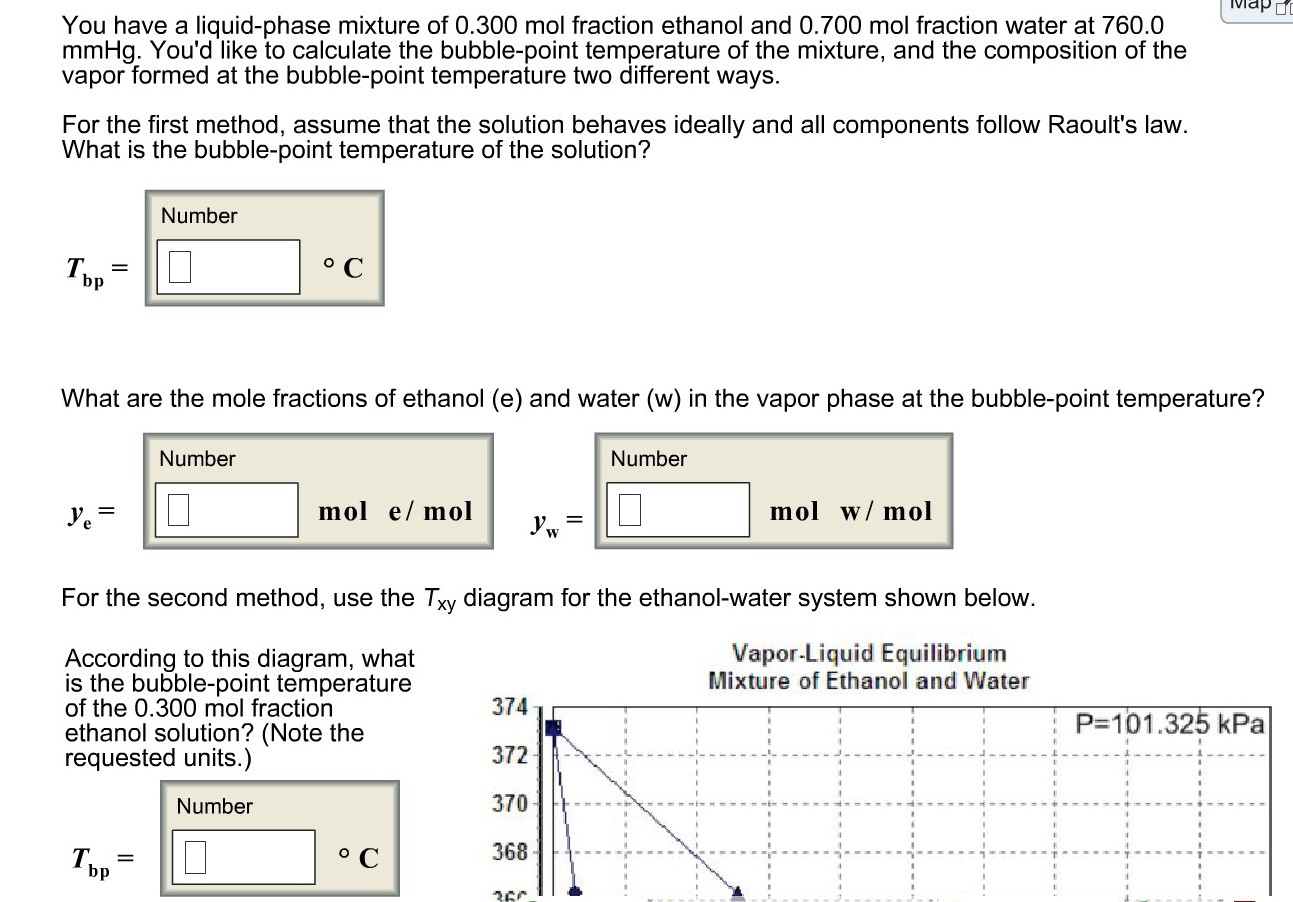
Graph of mole fraction and temperature against mixture fraction from... | Download Scientific Diagram

Radial profiles of mean mixture fraction, rms fluctuation of mixture... | Download Scientific Diagram
Fundamentals Of Combustion (Part 1) Dr. D.P. Mishra Department of Aerospace Engineering Indian Institute of Technology, Kanpur L

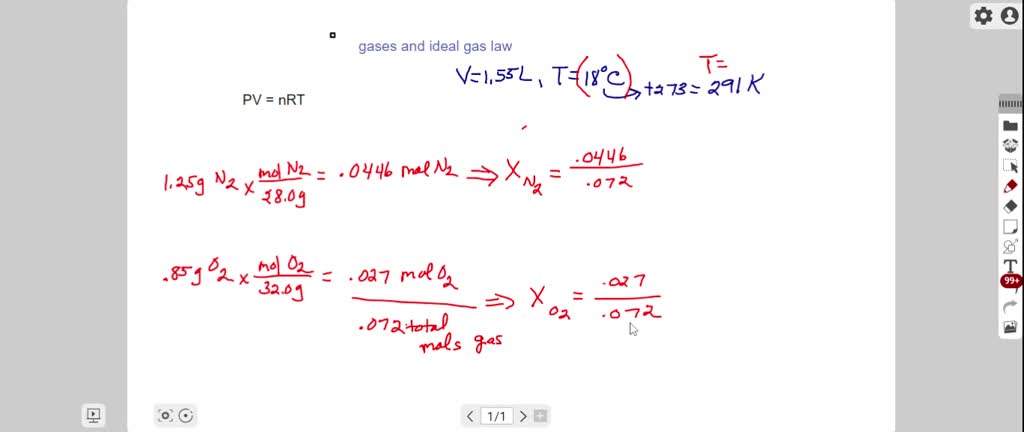
A mixture has 18 g water and 414 g ethanol. The mole fraction of water in mixture is (assume ideal behaviour of the mixture) :